Since the start of the pandemic, health authorities and life sciences companies have intensified their focus on risk management. While the European Medicines Agency published specific safety monitoring plans and guidance on risk management planning for COVID-19 vaccines, more broadly there has been a tighter focus on identifying and addressing safety concerns.
Yet nearly 10 years after becoming a mandatory document, the Risk Minimization Plan (RMP) is still poorly understood by many life sciences companies. Unlike required documents such as periodic safety update reports (PSURs), the Periodic Benefit-Risk Evaluation Report (PBRER), and clinical trial safety documents, the RMP remains a challenge for many in the industry. Companies have, therefore, been turning to experts, such as ProductLife Group’s experienced teams of RMP medical writers, for assistance.
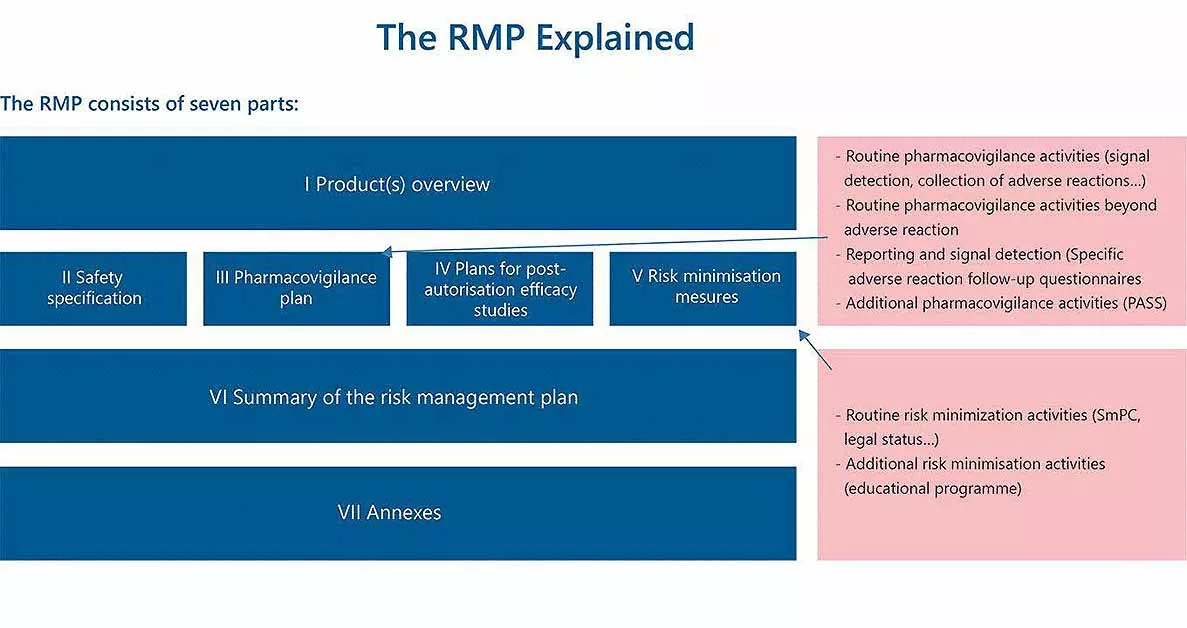
Understanding the RMP
The RMP is a document that details the organization’s risk management process. It presents the key safety concerns associated with the therapeutic product and the proposed activities to be implemented to monitor and manage them.
The RMP serves as an important tool for managing significant risks associated with a therapeutic product and may involve the implementation of risk minimization activities beyond product labelling to ensure that the benefits of the product outweigh its risks.
According to the European Union’s Good Pharmacovigilance Practices (GVP) guidelines, the objectives of an RMP are:
- To characterize, prevent and minimize the risks associated with a medicinal product
- To obtain more information on the benefit/risk ratio in certain populations excluded during the pre-authorization phase.
- To monitor the actual conditions of use and to evaluate the benefit-risk ratio of the drug in the general population.
The RMP is a dynamic document that should be updated throughout a product’s life cycle as knowledge about the product grows. For example, a safety concern that is initially categorized as an ‘important potential risk’ may be reclassified as an ‘important identified risk’ or removed from the RMP when new scientific or clinical data associated with the therapeutic product becomes available.
When to submit an RMP
As stated by the European Medicines Agency (EMA), a new or updated RMP may need to be submitted at any time during a product’s life cycle. Typical situations where an RMP are required include:
- At the time of a request for approval of new applications for products within the EU
- For products that don’t have an RMP, if a significant new safety concern or significant changes to the marketing authorization are identified
- At any time when there is a change in the list of the safety concerns or when there is a new or a significant change in the existing additional pharmacovigilance or additional risk minimization activities
- At the request of the health authorities
There are a number of key safety concerns that need to be included in an RMP. The first is an important identified risk that could have an impact on the risk-benefit balance of the product or have implications for public health. An identified risk is an untoward occurrence for which there is adequate evidence of an association with the medicinal product of interest.
Important potential risks also should be included in an RMP. This refers to any potential risk that could have an impact on the risk-benefit balance of the product or have implications for public health, including suspicion of association of the product and an adverse event such as drug class effect.
A third key safety concern is what is referred to as missing information, which describes gaps in knowledge about a medicinal product related to safety or use in particular patient populations, which could be clinically significant. These populations are typically those who have not been included in clinical trials, for example, pregnant women, children, and renally or hepatically impaired patients.
Why the RMP can be a challenge
For many life sciences companies, the RMP is a particularly challenging document to manage because it requires many different functions to collaborate, including safety teams, epidemiology, and sometimes medical affairs. Bringing these teams together is time consuming and can be hard to coordinate.
When an RMP is required for an older product, finding data can be extremely difficult since there are often no non-clinical or clinical studies, making it hard to establish a risk profile. Experienced professional RMP teams overcome these challenges by drawing on data from literature reviews and from medical expertise.
The issue of missing information from populations not represented in clinical trials presents a further challenge and again requires the expertise of physicians and pharmacists, as well as a literature review to address those knowledge gaps.
The RMP is a global requirement but there are country-specific minimization measures that present challenges for companies. RMP experts will typically adapt the tools and approaches depending on the local system. For example, the direct healthcare professional communication might need to be managed differently depending on the country to ensure that mandatory communication reaches HCPs.
Companies must also be ready to react to health authority requests, such as a request to mitigate medication confusion or switching a product from an over-the-counter drug to a prescription drug. There will be circumstances where a company wishes to push back against the regulator’s request, but to do so they need to understand the requirement and be ready with a compelling response.
How PLG supports clients
With an experienced and expanding team of medical writers in France, Italy, Central Europe, and India, PLG has the breadth and depth to help clients address all aspects of the RMP. Our teams comprise physicians, pharmacists, and Ph.D. scientists, bringing an extensive level of knowledge about therapeutic product risk management. Furthermore, having teams in different global locations and time zones ensures continuity of activities for clients.
PLG’s expert team handles RMP updates, RMP drafting, RMP review and validations, as well as advice to clients to guide them through the process and the RMP template. Our team also includes coordinators to develop the documents and organize data collection and other activities.
The RMP is a crucial and mandatory document, but many companies have struggled to adequately address the requirements. Working with a partner that has the expertise and knowledge to manage the RMP across multiple markets will ensure this crucial risk minimization measure is properly addressed.