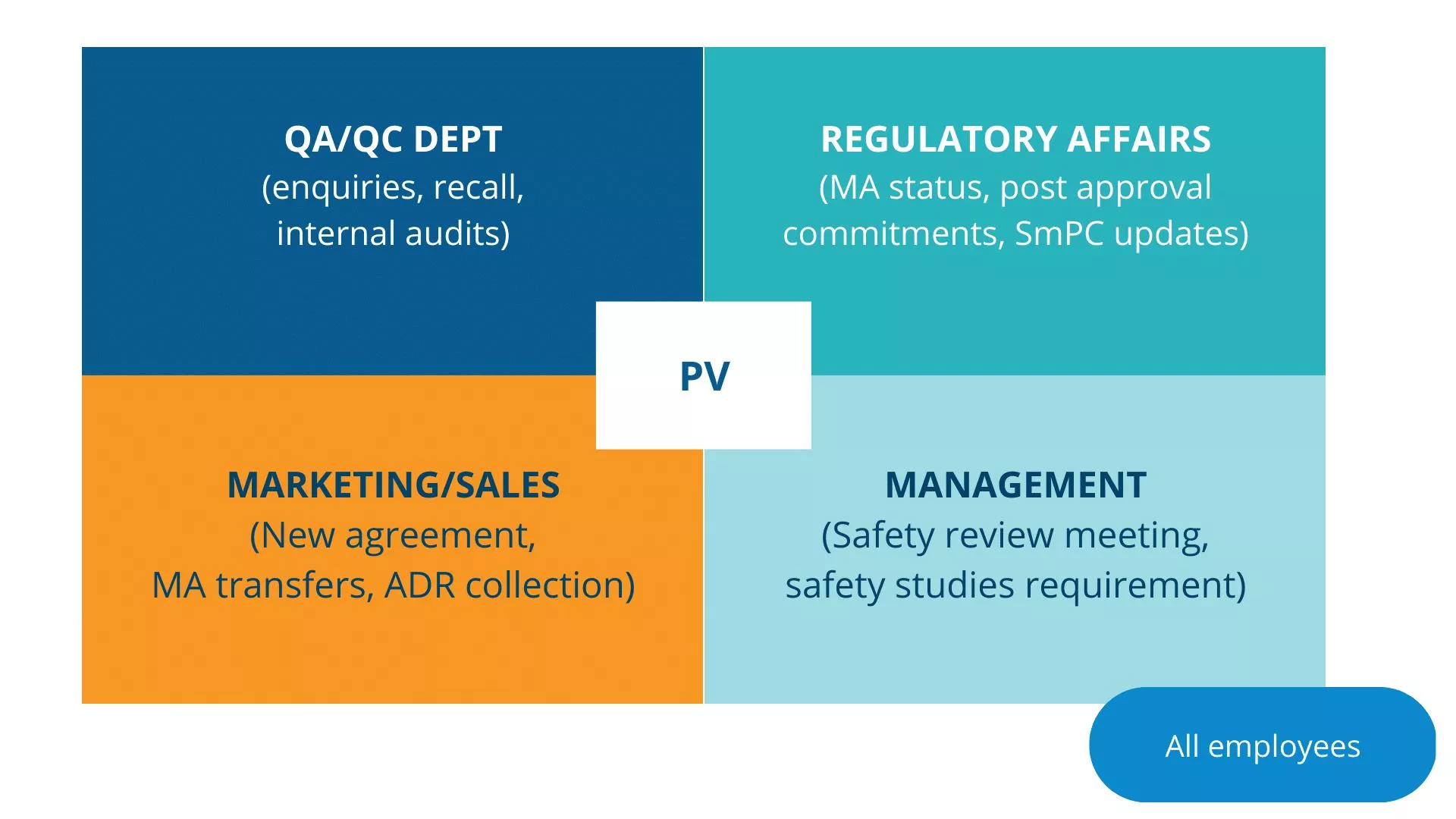
Pharmacovigilance is defined as science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem. It is the practice of monitoring the safety of pharmaceutical products during development phase and after they have been approved for use. It plays a crucial role in ensuring the ongoing safety and efficacy of medications, as adverse reactions and side effects can occur even after rigorous testing during clinical trials.
As such, cross-functional collaboration in pharmacovigilance is critical in ensuring that adverse events are identified and addressed promptly. This article will explore the importance of cross-functional pharmacovigilance collaboration and how it can be facilitated.