
UK National Contact Person for Pharmacovigilance
13 june 2023

A National Contact Person (NCP) for pharmacovigilance is an individual appointed by Marketing Authorization Holder (MAH) to oversee and coordinate pharmacovigilance activities within the United Kingdom. The NCP serves as a point of contact between MHRA and MAH.
After Brexit on 31st December 2020, MHRA guidelines suggested that each MAH appoint a QPPV who resides and operates in the UK. However, suppose MAH establish a QPPV that resides and operates in the EU/EEA. In that case, MHRA has given provision to appoint and nominate a national contact person (UK-NCP) for pharmacovigilance who resides and operates in the UK and reports to the QPPV. As per regulation 187 of the HMRs and the PSMF of the UK-authorised products, the UK-NCP should have access to the reports of suspected adverse reactions and UK PSMF. In addition, the UK-NCP should be the main point of contact and should be able to facilitate responses to pharmacovigilance queries raised by the MHRA, including via inspections.
Nomination of the UK-NCP:
Once the UK-NCP for pharmacovigilance has been appointed, their details should be notified to the MHRA via the MHRA submission Portal.
- The user/UK NCP logs on to the MHRA submissions portal using the below link:
https://mhrabpm.appiancloud.com/suite/
- Select ‘Medicines & E-Cigarettes’.

- Select the ‘Human Medicines’ tile.

- Select ‘National Contact Person for PV.’

- The UK NCP details will be captured in the below fields:
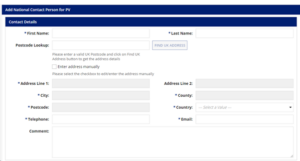
Accessing the MHRA Submission Portal
The MAH needs access to the MHRA submission portal and should be requested via the following steps on MHRA GOV. Once the MAH receives access to the MHRA submission portal, UK-NCP details are notified by filling in the necessary details, as shown in the figure above, for submitting details. MAH should receive a confirmation by email immediately upon completion of the form.
Appointment of Deputy for the UK-NCP
As per MHRA – “Guidance on the qualified person responsible for pharmacovigilance (QPPV) including pharmacovigilance system master files (PSMF)”, there is no requirement to appoint a deputy for the UK-NCP. However, appointing another person as the UK-NCP is necessary for extended absences greater than one month (such as maternity leave, long-term sick leave, etc.). The same should be notified to Licensing authority immediately and no later than 14 calendar days from the change. In practice, this means editing the existing details of the national contact person for pharmacovigilance that are saved in the MHRA Submissions portal. Again, there is no requirement for the UK-NCP to have 24/7 availability.
Support from PLG in Setting up PV Activities in the UK
PLG support MAH in setting up PV activities in the UK by offering UK QPPV or UK-NCP role. PLG support the UK-NCP role for MAH, whose QPPV resides and operates in EEA/EU. PLG has an experienced talent pool that resides and operates in the UK and has wide experience in UK PV regulation.
Register to our news and events
Go to our Events to register
Go to our News to get insights
