Key Figures

A complete set of local services for Life Science companies that we can scale globally
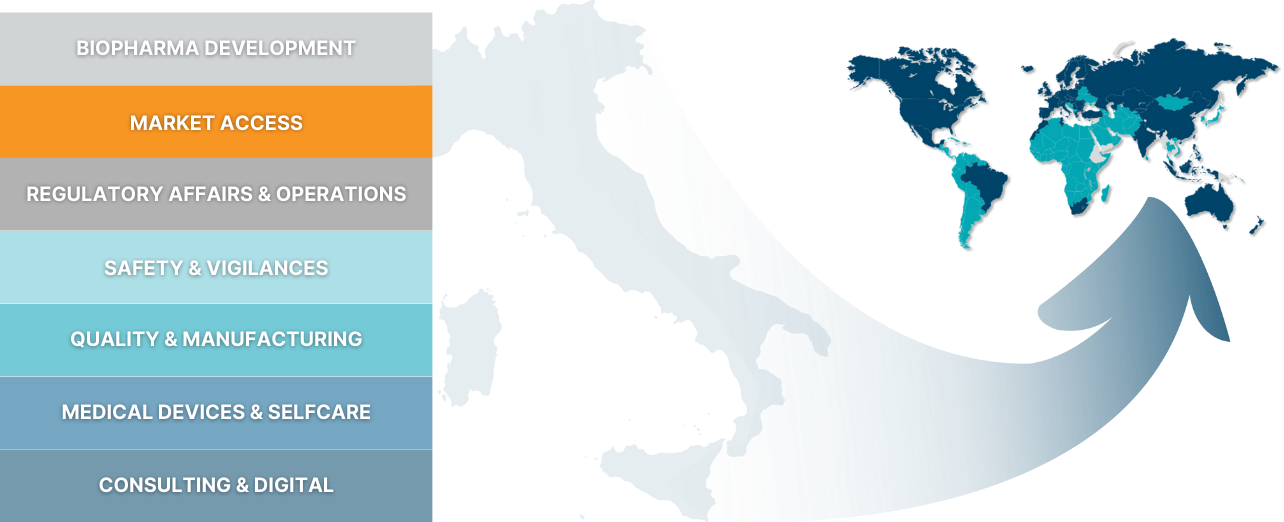
BIOPHARMA DEVELOPMENT
- CMC Development
- Regulatory Consulting
- QA Consulting (including QP Release Services)
MARKET ACCESS
- Market Access Strategy
- P&R and HTA strategy & support
- HEOR
- Patient Access & RWD/RWE
- Research & Intelligence
- Patient Programs
REGULATORY AFFAIRS & OPERATIONS
- Non-clinical Development
- Clinical Development
- Regulatory Strategy Services
- Regulatory Writing
- Regulatory Operations
- Regulatory Lifecycle Services
- Regulatory CMC
- Marketing Authorization Services
SAFETY & VIGILANCES
- Medical Information
- Local literature monitoring
- End to End Safety & Vigilances
- GSO
- Safety Writing & Literature Monitoring
- Case Management
QUALITY & MANUFACTURING
- Pre-Market Quality Services
- Market Authorization Holder and Local Affiliate Services
- Manufacturing Services
- Audit & Training
- Non GxP Compliance Management
- Engineering
- Qualification & Calibration
- Facility management-Energy & Waste
- Digital & Quality Compliance
MEDICAL DEVICES & SELFCARE
- Medical Devices
- Nutrition
- Cosmetic & Dermatological Solutions
CONSULTING & DIGITAL
- Strategy Consulting
- Consulting in Operations
- Digital Consulting
- Digital Delivery
- Digital Operations
- Digital Quality & Compliance
C&P Engineering

C&P Engineering is a multidisciplinary engineering and consulting firm specializing in providing tailored solutions for the pharmaceutical, medical, nutraceutical, and cosmetics industries. Founded in 2005, the company supports clients in optimizing industrial processes while adhering to regulatory compliance standards like EU and FDA cGMPs.
These services collectively ensure that clients receive a tailored, client-centric approach ensuring cost-effective, high-quality, and compliant solutions across engineering and operational domains.
C&P Engineering is certified ISO 9001: 
Intexo

Intexo specializes in regulatory affairs and patient access within the healthcare sector, combining technical expertise with a strong commitment to societal values:
Development of negotiation strategies, pricing and reimbursement dossiers, and innovative communication projects designed to support product launch and accessibility.
Combination of strategic consulting and regulatory affairs to help healthcare companies navigate complex regulatory frameworks. Design of forward-thinking strategies to manage shifting compliance standards, optimize market-entry processes, and align operations with long-term regulatory goals.
As a certified B-Corp, Intexo integrates compliance with quality assurance systems, promotes gender equality, and upholds environmental and social responsibility throughout its operations.
Intexo’s combined technical and ethical framework supports healthcare companies in achieving regulatory milestones while advancing the shared goal of accessible and sustainable healthcare solutions. They emphasize collaboration with clinicians, patient associations, and scientific communities, while advocating for compliance, quality systems, and gender equality in their operations.
Intexo is certified:



LifeBee

LifeBee is a Consulting and Digital boutique firm, supporting Life Science companies on their path towards Digitalization, Operational Excellence, and Compliance with Customized Advisory and innovative Digital Solutions. The focus is on GxP regulated areas such as Laboratories, Logistics, Manufacturing, Serialization, Pharmacovigilance, Quality Assurance, Regulatory Affairs, Project & Portfolio Management of Biopharmaceutical Companies, Medical Devices, and Nutraceuticals.
LifeBee delivers a complete suite of consulting and digitalization services that optimize life science companies’ operations, ensuring compliance while driving transformation. Their tailored solutions allow businesses to streamline processes, reduce risks, and stay competitive by leveraging both strategic advice and innovative digital tools across every phase of product development.
LifeBee is certified: 

PEC – Pharma Education Center

- Diverse Training Modalities: PEC’s educational portfolio includes on-demand, scheduled, and in-house training sessions. These are customized to cover essential topics such as compliance with international standards (e.g., GMP, ISO), risk management, and the implementation of cutting-edge pharmaceutical technologies.
- Knowledge-Sharing Events: The center organizes industry-focused events featuring thought leaders who share the latest developments in science, technology, and regulations. These events provide networking opportunities and foster collaboration among professionals.
- Expert-Led Programs: Courses are designed and conducted by industry experts with extensive field experience, ensuring that participants gain practical insights in addition to theoretical knowledge.
- Benefits to Organizations: PEC helps companies enhance workforce competence, align operations with regulatory requirements, and build internal capabilities to remain competitive in a globalized market.
Pharma Education Center stands out by delivering flexible, up-to-date, and customized learning opportunities that empower professionals to address industry challenges effectively while contributing to their organizations’ growth.
Pharma D&S

Pharma D&S is a comprehensive consulting firm specializing in the pharmaceutical, biotech, and medical device industries. It provides tailored solutions to support clients throughout the entire product lifecycle, from research and development to post-marketing activities. Its key services include:
Pharma D&S offers a distinctive value proposition through its highly customizable approach, acting as an extension of client teams, and delivering compliance-focused, efficient solutions tailored to specific needs. Its broad expertise ensures comprehensive support in achieving both regulatory and operational goals.
Pharma D&S is certified:



CONTACT US
By email: [email protected]
ProductLife Group
Corso Italia, 17 – 20122 Milan
PLG Italy, LifeBee, Intexo
Piazza Luigi di Savoia 22 – 20124 Milan
C&P Engineering
Via dei Pratoni, 16 – 50018 Scandicci (FI)
Regus, Piazza Carlo Magno, via Tiburtina 21 – 00162 Rome
Via Roma, 25 -41037 Mirandola (MO)
Centro Direzionale Colombirolo Via Roma 74 – 20060 Cassina de Pecchi (MI)
Talent Garden Genova, Giardini Baltimora – 16121 Genova
Via Funicolare, 4 – 6912 Pazzallo (Lugano) Switzerland
PEC – Pharma Education Center s.r.l.
Via dei Pratoni, 16 – 50018 Scandicci (FI)
Pharma D&S
Via dei Pratoni, 16 – 50018 Scandicci (FI)
Centro Direzionale Colombirolo Via Roma 74 – 20060 Cassina de Pecchi (MI)
Via Gualtiero Serafino, 8b – 00136 Rome
Centro Direzionale Isola E7, E5 – 80143 Naples






