The Drug approval processes in the United States (USA) & Europe are the most demanding in the world.
North America (NA) and Europe follow different approval procedures and each of them is unique in its own way. Although they share the common goal of patient safety and preserving public health, there are notable differences in the regulations, the functions of the regulatory authorities, drug approval process.
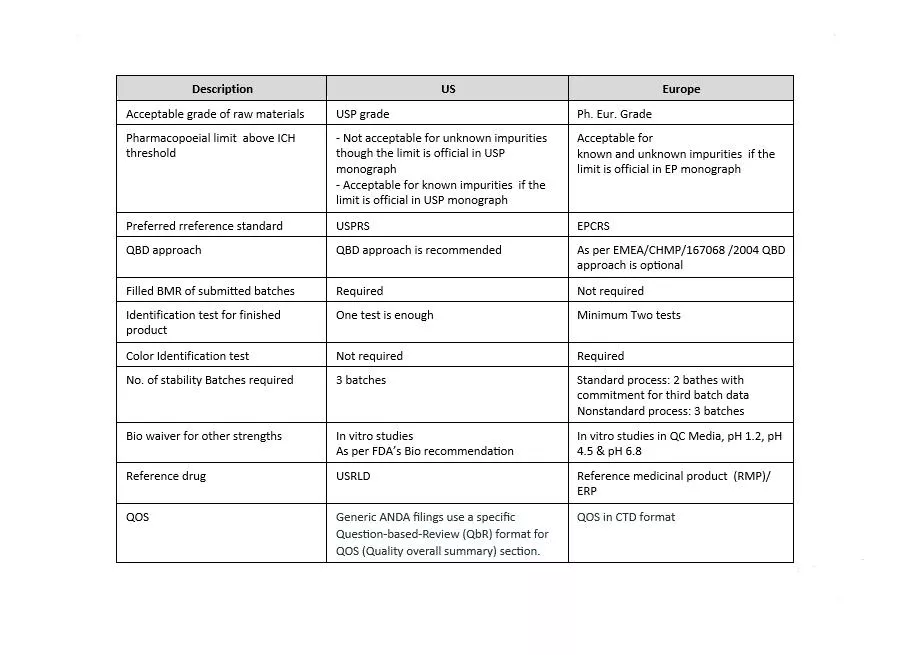
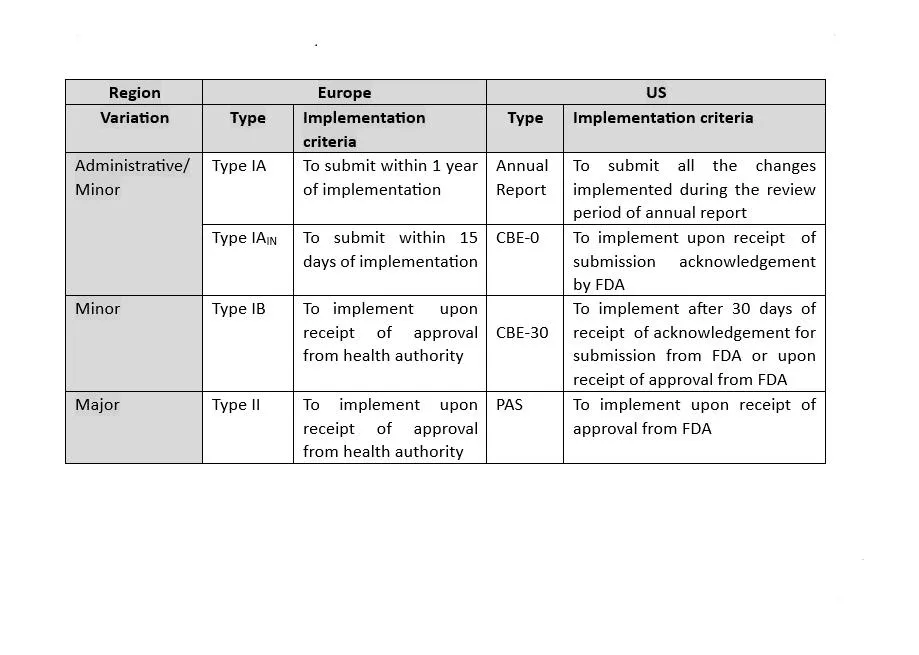
The format for chemistry, manufacturing and control (CMC) information is presented in ICH guideline M4. US and Europe are both Founding Regulatory Members of the ICH Association. As both regions are members of the ICH, they share many scientific and regulatory guidelines. These guidelines are incorporated in the US as Guidance for Industry and Notice to Applicants in Europe. Also, both of these health authorities continuously make efforts to harmonize their compendial monographs’ content. Despite all these efforts, there are still some specific requirements which are unique for the respective region.
Let’s have an overview of the differences between the US and Europe’s regulatory expectations. First, we need to understand why there are differences in regulatory expectations. The main reason for the existence of significant differences in requirements for both regions are:
- How the regulatory guidelines are interpreted and implemented in these regions.
- The practical consideration of guidelines is significantly different in both regions.
- These differences originate from the varying circumstances faced by individual authorities and the mindsets of the authorities.
- The tradition and legislation already in place before the harmonization processes have also played a significant role in the Agency’s current expectations.
- Some historically grown differences greatly influence the presentation, anticipated content and terminology of the application dossier in both regions.