
Electronic Product Information (ePI)
13 october 2021

The last few years have been characterized by strong push towards digitalization, increased during the pandemic.
The European regulatory is going to align with the digitization wave, focusing its attention on Product Information, as first priority.
Why create an Electronic Product Information (ePI)?
Based on Article 59 (4) of Directive 2001/83 EC, the shortcomings of the current Product Information format have been analyzed and ways to improve it have been defined, trying to meet the needs of all PI users, from patients to professionals.
So, EMA in collaboration with HMA and EC has decided to develop a Standard electronic format of SmPC and Leaflet (ePI).
After gathering the main ideas and key points, which should guide the implementation of the ePI in Europe, EMA and some European countries, such as Spain and Sweden, have already started adopting a semi-structured electronic version of the document.
The digitization would allow the document to be updated effectively, reducing the risk of errors, would allow the addition of multiple digital contents to help the patient get more information.
The electronic version will always guarantee safe, reliable and protected content and data and it will complete the paper version that will not be discontinued.
The starting point, therefore, is the creation of a semi-structured and standardized ePI for the whole of Europe, which combines SmPC and Leaflet.
The insights from all the stakeholders across the EU are being gathered through an online survey consultation: https://ec.europa.eu/eusurvey/runner/ePIStandardConsultation
A first draft of common standard prototype is planned to be released in summer 2021 and the publication of the complete prototype is expected in October 2021.
The implementation process of the ePI in the European Union will take place with different timelines and methods, due to different resources and priorities of the individual Member States.
Based on this approach, the digitization of the package leaflet, is a real hot topic.
Many associations are collaborating to collect the main information and characteristics that an electronic leaflet should have and its possible applications.
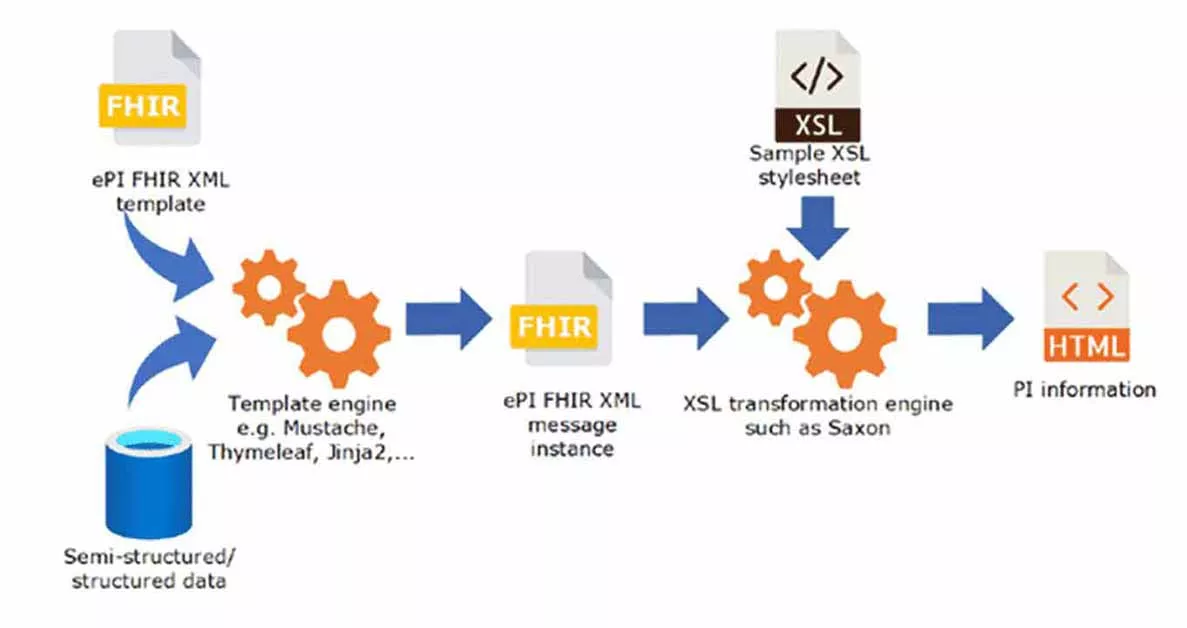
Fig. 1 – Transformation of PI information, in https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/draft-eu-common-standard-electronic-product-information-human-medicines-epi_en.pdf
The main purpose of the digitization of the package leaflet is to overcome the criticalities of the paper format, especially in terms of readability and accessibility, using technology for the benefit of the patient, adding personal and specific information on therapies, integrating functions that can help more patients, even with disabilities, to always have safe and certified information to support their therapy, and make them actively involved in their care.
The entire pharmaceutical and healthcare world agrees on the extreme need to bring such an important document for the patient into a form that is easy to read and to consult by all the users.
Know more information about the cPI draft by EMA in https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/draft-eu-common-standard-electronic-product-information-human-medicines-epi_en.pdf
If you need to learn more on the importance of regulatory intelligence in the pharmaceutical sector, please feel free to contact ProductLife Group at [email protected]
Silvia Belelli, Regulatory Affairs Officer Product-Life Group Italy
Register to our news and events
Go to our Events to register
Go to our News to get insights
