
USFDA Guidance released! For Product-Specific Guidance Meetings for the ANDA applicants
25 april 2023

Product-Specific Guidance Meetings for Generic Drug Development: Challenges
USFDA releases Product-Specific Guidance (PSG) for the development of generic drugs. PSG provides recommendations for developing generic drug products and describes the FDA’s current thinking on the evidence needed to demonstrate that an ANDA is therapeutically equivalent to a specific reference-listed drug (RLD). However, many a time, generic drug developers face problems in developing their products due to
- No product-specific guidance for their target product.
- Studies suggested that product-specific guidance may be difficult to conduct.
- Alternate pathway (in vitro or in vivo) for demonstrating equivalence to RLD.
Thus, there is a need for Product-Specific Guidance Meetings Between FDA and ANDA Applicants Under GDUFA to have a clear development pathway for further product development. This guidance gives information on meeting types, procedures, and resolutions.
Types of PSG Meetings Between Applicants and FDA Under GDUFA
As per guidance, three types of PSG meetings occur between applicants and FDA:
- PSG teleconferences (which include pre and post-submission PSG teleconferences). PSG teleconferences would be needed when the applicant has already commenced (i.e. the study protocol was signed by the study sponsor and/or the Contract Research Organization) or completed an in vivo BE study. Suppose an applicant seeks further feedback from FDA after a PSG teleconference to ensure that any proposed changes or additions to an applicant’s in vivo study would result in an approach that complies with the relevant statutes and regulations. In that case, the applicant may request a pre-submission PSG meeting or a post-submission PSG meeting.
- Pre-submission PSG meetings- A prospective ANDA applicant can request a pre-submission PSG teleconference before submission of the ANDA in the following case:
The ANDA has not yet been submitted when the FDA publishes a new or revised PSG that introduces or revises a recommendation related to an in vivo BE study. This is because the prospective ANDA applicant has already commenced an in vivo BE study as of the published date for the new or revised PSG (i.e. the study protocol was signed by the study sponsor and/or the Contract Research Organization before the PSG publication date).
- Post-submission PSG meetings- An ANDA applicant can request a post-submission PSG teleconference if the ANDA has been submitted in one of the following cases:
When FDA publishes a new PSG, which includes a recommendation to conduct an in vivo BE study, the ANDA applicant did not conduct an in vivo BE study.
When FDA publishes a revised PSG which includes a recommendation to conduct an in vivo BE study, the previous PSG did not include a recommendation to conduct an in vivo BE study, and the ANDA applicant commenced or completed the in vitro BE study or studies either that were recommended by FDA in the previous PSG or that the ANDA applicant decided to pursue after a previous product development meeting.
Guidelines for Pre-Submission and Post-Submission PSG Meetings for ANDA Applicants Under GDUFA
The pre-submission PSG teleconference and the subsequent pre-submission PSG meeting should occur before submission of the ANDA (i.e., within the pre-submission phase) so that the prospective ANDA applicant obtains FDA’s feedback on an approach other than the approach recommended in the PSG before submission of the ANDA. ANDA applicant is eligible to have a post-submission PSG meeting if it first requests and has a post-submission PSG teleconference with FDA. The post-submission PSG teleconference and the subsequent post-submission PSG meeting should occur before responding to a possible BE deficiency identified in a Discipline Review Letter (DRL) or a BE deficiency identified in a Complete Response Letter (CRL). ANDA applicants should submit a request for a PSG teleconference within 60 days after the publication of the new or revised PSG so that FDA can provide timely feedback to applicants. Applicants can request a PSG teleconference more than 60 days after publishing the new or revised PSG. However, the 30-day time frame for conducting PSG teleconferences is only applicable for complete packages submitted within 60 days after publication of the PSG.
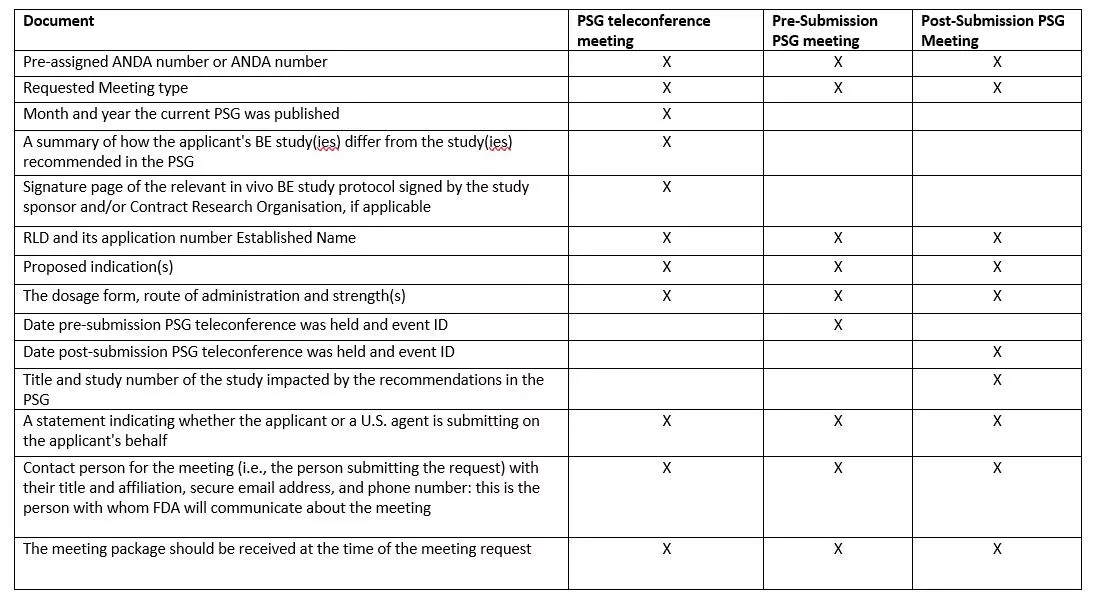
Documents Required for Pre-Submission and Post-Submission PSG Meetings
With the pre-submission PSG teleconference request, a prospective ANDA applicant should submit the title page, protocol summary, and the signature page of the relevant in vivo BE study protocol signed and dated by the study sponsor and/or the Contract Research Organization.
The information required for different types of meetings are:
FDA Meeting Request and Timeline Guidelines
For pre-submission and post-submission PSG meetings, FDA agreed to grant or deny the meeting request within 14 days after FDA received the request. If granted, FDA agreed to hold the pre-submission PSG meeting within 120 days after FDA received the request, whereas, for post-submission SG, it is within 90 days after FDA received the request. FDA intends to issue the official, finalized minutes to the applicant within 30 days after the PSG teleconference, pre-submission PSG meeting, or post-submission PSG meeting. FDA recommends that the applicant submit its concerns about the meeting minutes in writing to FDA within ten calendar days of receipt of the official meeting minutes.
Importance of Product-Specific Guidance Meetings for ANDA Applicants
USFDA had released guidance for product-Specific Guidance Meetings Between FDA and ANDA Applicants Under GDUFA. This guidance helps an ANDA applicant troubleshoot problems arising during and after the generic drug development before getting a complete response letter. In addition, this guidance further strengthens the interaction of the USFDA with an ANDA applicant.
Ref- Product-Specific Guidance Meetings Between FDA and ANDA Applicants Under GDUFA, February 2023
Register to our news and events
Go to our Events to register
Go to our News to get insights
